-
Notifications
You must be signed in to change notification settings - Fork 75
Vocab. LOINC
Logical Observation Identifiers Names and Codes (LOINC) is a standardized coding system for laboratory and clinical tests, procedures, observations, surveys, and corresponding results.
All LOINC source information is obtained from the following:
1. LOINC Website that is supported by the Regenstrief Institute:
- LOINC Table Core (Observations and Measurements)
- LOINC Answers
- LOINC Multiaxial Hierarchy
- LOINC Document Ontology
- LOINC Panels and Forms
- LOINC Groups
- LOINC to SNOMED mapping
- LOINC Replacement Mapping
2. The National Library of Medicine (NLM) Website:
- LOINC To CPT4 Mapping
- Manual table of LOINC Classes
- Values of a “long_common_name” field are considered to be preferred terms for concept names.
- Values of “consumer_name”, “shortname” and “relatednames2” fields reside in the CONCEPT_SYNONYM table as synonyms.
| Source Concept Type | Standard Concept |
| LOINC Observations, Measurements and Procedures | Standard |
| LOINC Answers | Standard |
| LOINC Hierarchy | Classification |
| LOINC Classes | Classification |
| LOINC Categories | Classification |
| LOINC Groups | Classification |
| LOINC Parts (Component, Method, Property, Scale, System, Time) | non-Standard |
LOINC Concepts are located in such domains as “Observation”, “Measurement”, “Procedure” and “Meas Value”.
Previously all concepts with a LOINC property of “classtype” with values “1” and “2” were considered to be Measurements. However, an initial analysis of such extended LOINC properties as “property”, system”, “scale_type”, “class”, “method_typ”, “definitiondescription” and “survey_quest_text” revealed that the LOINC domain distribution can be more accurate if used the combinations of LOINC properties. In turn, the Concept Class definition is connected with a defined Domain.
So, they were assigned pursuant to the following criteria:
| Source | Dependencies | Domain | Concept Class |
| Loinc core Table | A “classtype” field value = 1 (Laboratory class) or 2 (Clinical class) AND:
1. A “survey_quest_text” field value contains a question mark, which always defines LOINC questions. OR 2. A “scale_typ” field has “Set” value indicating concepts used for Clinical Attachments only. OR 3. A “property” field value contains: • “Hx” - History of different clinical and administrative events • “Anat” - Anatomical sites, points, body structure descriptions • “ClockTime”, “Date”, “TmStp”, “TmStpRange”, “DateRange” - time/date/date and time/ranges of different clinical and administrative events • “Desc” - Description of procedures or clinical events • “Instrct” - Instructions • “Loc” - Location • “Pn” - Person Name - administrative information about a person • “Addr” - Address • “EmailAddr” - Email Address • “Tele” - Telephone Number • “Xad” - also Address • “Txt” - Text - notes and comments • “URI” - Uniform Resource Identifier • “Bib” - Bibliographic Citation OR 4. A “property” field value is “ID” representing Identifiers such as serial numbers, codes and IDS while “system” field value in “BPU” (Blood Product Unit - additional information about a blood transfusion), “^Patient” (patient related codes), “Vaccine” (vaccination-related additional information). OR 5. Values of a “system” field are represented by the following: • “^Family member”,“Community”, “^Brother”,“^Daughter”, “^Sister”, “^Son” - observational particular person-related data • “^CCD” - Continuity of Care Document • “^Census tract” - information about people in a geographic region defined for the purpose of taking a census • “^Clinical trial protocol” - Clinical trial protocol documentation and reports • “*” - Mixed category defining sites, types, locations, device peculiarities, etc. • “^Contact”, “^Emergency contact” - contact information • “^Donor” - a donor type and ID • “^Event” - information about incidents or events • “^Facility” - information about services • “Provider” - provider-related information • “Report” - report specification • “Repository” - health data repository • “School” - a name of a school • “Surgical procedure” - surgical specification OR 6. values of a “system” filed are “^Patient” and “*^Patient” which contain observational data directly linked with a patient and, simultaneously, values of a “scale_typ” field are in: • “Doc” - various types of documentation • “Nar” - narrative text • “Nom” - nominal or categorical responses that do not have a natural ordering • “Ord” - ordered categorical responses, e.g. “Yes”, “No”. while a “method_typ” field value is not “Apgar” (Apgar score is a screening test used to measure the vital signs of a newborn) OR 7. values of a “property” field are in: • “Imp” - impression/interpretation of a study - is used to represent a property when the evaluation is a mental abstraction based on one a collection of measurements and/or data. • “Find” - findings (seem to be Observations in all cases regardless of the “system”, but not) • “NRat” - number = count/time - e.g. “How many cigars are you smoking per week now?” • “Num” - number - e.g. “[#] Pregnancies” • “PrThr” - the presence of symptoms, historical facts, statuses, etc. • “RelRto” - relative ratio - e.g. relative risk of developing a disease • “Time” - time aspects - age, hours, years, etc. • “Type” - a mixed category with a general notion of something • “Arb” - arbitrary - a mixed category, e.g. “Informed consent obtained” or “RhoGam candidate” while values of a “class” field are not “COAG” (it includes laboratory, tests related to hemostasis) and “PULM” (it is about measurements of respiratory function). A general exclusion criterion: a “long_common_name” field value contains the words as “scale” or “score” (they indicate “Measurement” domain). |
Observation | Clinical Observation |
| OR
8. class = ‘RAD’ - radiology - represents radiology procedures like CT, XR, MR and so on Exclusion: a “partnumber” field = LP7753-9 (Qn) or LP200093-5 (Calcium score) or LP200395-4 (Densitometry) |
Procedure | Clinical Observation | |
| Other cases when a “classtype” field value = 1 (Laboratory class) | Measurement | Lab Test | |
| Other cases when a “classtype” field value = 2 (Clinical class) | Measurement | Clinical Observation | |
| A “classtype” field value = 3 (Claims attachment) | Observation | Claims Attachment | |
| A “classtype” field value = 4 (Surveys) | Observation | Survey | |
| LOINC Hierarchy | A “Code text” field value contains the following:
• “identifier”, “ID”, “number”, “name”, “age”, “status”, “provider”, “subject”, “device”, “version”, “ICD code”, “coding system”, “priority” • “time”, “date”, “date and time”, “term” • “comment”, “narrative”, “note”, “directive”, “attachment”, “reference”, “interpretation”, “summary”, “information”, “suggestion”, “administration”, “route”, “instruction”, “intention”, “consent”, “report”, “flowsheet”, • “recommended”, “reported”, “received”, “requested”, “reviewed”, “ordered”, “available”, “issued”, “performed”, • “lifetime risk”, “risk factor” “alert”, “known exposure”, “not yet categorized”, “reason for” At the same time a “code_text” field value does not contain: “thrombin time”, “clotting time”, “bleeding time”, “clot formation”, “kaolin activated time”, “closure time”, “protein feed time”, “Recalcification time”, “reptilase time” ,”russell viper venom time”, “implanted device”,” dosage.vial”, “isolate”, “within lymph node”, “cancer specimen”, “tumor”, “chromosome”, “inversion”, “bioavailable”. |
Observation | LOINC Hierarchy |
| A “path_to_root” field includes LP29684-5 (Radiology) | Procedure | LOINC Hierarchy | |
| Other values of a “code_text” field | Measurement | LOINC Hierarchy | |
| LOINC Classes | LOINC Class name contains such the words as “history”, “report”, “document”, “miscellaneous”, “public health” | Observation | LOINC Class |
| Other cases | Measurement | LOINC Class | |
| LOINC Group | A “parentgroupid” field = LG85-3 (Radiology) or LG41849-7 (Region imaged: Lower extremity) or LG41814-1 (LG41814-1) | Procedure | LOINC Group |
| Other cases | Measurement | LOINC Group | |
| LOINC Answer | Independently | Meas Value | Answer |
Relationships are defined within LOINC and between LOINC and CPT, and LOINC and SNOMED:
A purpose of Internal LOINC relationships is to build a LOINC Hierarchy, which makes it easier to sort and browse the database.
| Relationship (reverse relationship) | From | To |
| Maps to
(Mapped from) |
Each LOINC concept | The same LOINC concept |
| Is a
(Subsumes) |
Descendant LOINC Class | Ancestor LOINC Class |
| Is a
(Subsumes) |
LOINC Group | LOINC Group Category |
| Is a
(Subsumes) |
LOINC Measurement or Observation | LOINC Class |
| Is a
(Subsumes) |
LOINC Measurement or Observation | LOINC Group |
| Is a
(Subsumes) |
Descendant LOINC Measurement or Observation | Ancestor LOINC Measurement or Observation |
| Panel contains
(Contained in panel) |
LOINC Panel | LOINC Measurement or Observation |
| Has Answer
(Answer of) |
LOINC Question | LOINC Answer |
| Has type of service
(Type of service of) |
LOINC Measurement or Observation | LOINC concept indicating a Type of Service (Document Ontology) |
| Has subject matter
(Subject matter of) |
LOINC Measurement or Observation | LOINC concept indicating a Subject Matter Domain (Document Ontology) |
| Has role
(Role of) |
LOINC Measurement or Observation | LOINC concept indicating a Role (Document Ontology) |
| Has setting
(Setting of) |
LOINC Measurement or Observation | LOINC concept indicating a Setting (Document Ontology) |
| Has kind
(Kind of) |
LOINC Measurement or Observation | LOINC concept indicating a Kind of Document (Document Ontology) |
| Concept replaced by
(Concept replaces) |
Upgraded LOINC concept | New LOINC concept |
| Has component (Component of) |
LOINC Measurement or Observation | LOINC Component |
| Has method (Method of) |
LOINC Measurement or Observation | LOINC Method |
| Has property (Property of) |
LOINC Measurement or Observation | LOINC Property |
| Has scale type (Scale type of) |
LOINC Measurement or Observation | LOINC Scale |
| Has system (System of) |
LOINC Measurement or Observation | LOINC System |
| Has time aspect (Time aspect of) |
LOINC Measurement or Observation | LOINC Time |
The purpose of External LOINC relationships is the following: to simplify a cross-analysis of clinical information systems, laboratory information, specifications or standards with a context of combined use of the LOINC and such a coding system as either the SNOMED or CPT4, or PPI.
| Relationship | From | To | Description | Count |
| LOINC - SNOMED eq | LOINC Lab Test | SNOMED concept of 705114005 “LOINC Code System” (Qualifier Value) | An identifier for a LOINC referenced component according to the "Using LOINC with SNOMED CT" guide. Note, this is not a mapping! | 22560 |
| LOINC - SNOMED eq | LOINC Hierarchy | SNOMED Body Structure (the “Spec Anatomic Site” domain) | Defines a body structure observed or measured | 26 |
| LOINC - SNOMED eq | LOINC Hierarchy | SNOMED Morph Abnormality (the “Observation” domain) | Indicates present in certain disease states structures which observed or measured | 3 |
| LOINC - SNOMED eq | LOINC Hierarchy | SNOMED Observable Entity (the “Observation” domain) | Represents an entity observed or measured | 8 |
| LOINC - SNOMED eq | LOINC Hierarchy | SNOMED Organism (the “Observation” domain) | Defines a microorganism measured | 116 |
| LOINC - SNOMED eq | LOINC Hierarchy | SNOMED Substance (domains of “Device” and “Observation”) | Represents a biological substance or pharmacological agent measured | 849 |
| LOINC - SNOMED eq | LOINC Hierarchy | SNOMED Specimen (the “Specimen” domain) | Indicates a specimen source observed or measured | 4 |
| LOINC - CPT4 eq | LOINC Lab Test | CPT4 Procedure or Measurement | Defines link from a LOINC concept to a less granular CPT4 concept | 1886 |
| Mapped from | LOINC Survey/Clinical Observation/Answer | PPI Observation or Measurement | Indicates an equivalent mapping from a concept of the PPI questionnaire-like vocabulary to a corresponding LOINC concept | 2155 |
All internal “Is a” and “Panel contains” relationships participate in the creation of the CONCEPT_ANCESTOR table, creating an internal hierarchy within the LOINC.
IIIa. OMOP CDM hierarchical crosswalk between LOINC and SNOMED was built by the following sequence of actions:
1. Assemblage of the pool of attributes for Standard fully-defined SNOMED Expressions of the Measurement Domain.
2. Connection of SNOMED Attributes to LOINC Laboratory Procedures using LOINC Parts and modified LOINC/SNOMED CT Collaborative Files.
3. Multi-axial matching of sharable attributes in different combinations for Standard LOINC and SNOMED Measurements (see Figure 1).
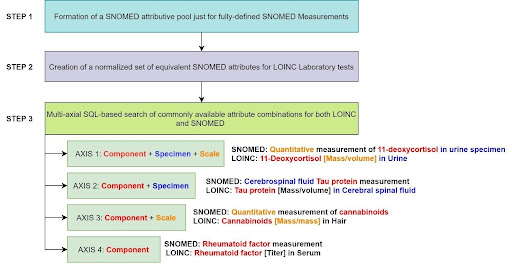
Figure 1. The methodology of an automated hierarchical crosswalk between LOINC Laboratory tests and SNOMED CT Measurements.
The SQL script for the crosswalk creation is available in the OHDSI GitHub Repository as the part of the LOINC load stage (steps 17-22). As a result, there are 16,5K hierarchical links between LOINC Laboratory Tests and SNOMED Measurements.They are represented by “Is a” relationships (with reverse of “Subsumes”) which semantically embed LOINC Laboratory tests, as a descendant, “under” SNOMED Measurements in the OMOP Hierarchy of Measurements (see tables 1 and 2).
Table 1. The extract from the hierarchical crosswalk for more granular LOINC Laboratory tests, which are connected to a generic SNOMED Measurement through the “Is a” relationship.| loinc_code | loinc_name | relationship_id | snomed_code | snomed _name |
| 69941-3 | Glucose [Moles/volume] in Serum or Plasma --20 minutes post dose lactose PO | Is a | 33747003 | Glucose measurement, blood |
| 69942-1 | Glucose [Moles/volume] in Serum or Plasma --40 minutes post dose lactose PO | Is a | 33747003 | Glucose measurement, blood |
| 69943-9 | Glucose [Moles/volume] in Serum or Plasma --20 minutes post 50 g lactose PO | Is a | 33747003 | Glucose measurement, blood |
| 69944-7 | Glucose [Moles/volume] in Serum or Plasma --40 minutes post 50 g lactose PO | Is a | 33747003 | Glucose measurement, blood |
| 70208-4 | Glucose [Moles/volume] in Serum or Plasma --pre 100 g glucose PO | Is a | 33747003 | Glucose measurement, blood |
| 72895-6 | Glucose [Moles/volume] in Serum or Plasma --2.5 hours post dose lactose PO | Is a | 33747003 | Glucose measurement, blood |
| 72896-4 | Glucose [Moles/volume] in Serum or Plasma --15 minutes post dose lactose PO | Is a | 33747003 | Glucose measurement, blood |
| 74084-5 | Glucose [Moles/volume] in Serum or Plasma --15 minutes post 50 g lactose PO | Is a | 33747003 | Glucose measurement, blood |
| 74774-1 | Glucose [Mass/volume] in Serum, Plasma or Blood | Is a | 33747003 | Glucose measurement, blood |
| 75405-1 | Glucose [Moles/volume] in Serum or Plasma --1.5 hours post dose triple bolus | Is a | 33747003 | Glucose measurement, blood |
| 75637-9 | Glucose [Moles/volume] in Serum or Plasma --45 minutes post 50 g lactose PO | Is a | 33747003 | Glucose measurement, blood |
| 77677-3 | Glucose [Moles/volume] in Serum, Plasma or Blood --2 hours post meal | Is a | 33747003 | Glucose measurement, blood |
| 77681-5 | Glucose [Moles/volume] in Serum or Plasma --11 hour post XXX challenge | Is a | 33747003 | Glucose measurement, blood |
| 77135-2 | Glucose [Moles/volume] in Serum, Plasma or Blood | Is a | 33747003 | Glucose measurement, blood |
| 93791-2 | Glucose [Mass/volume] mean in Serum or Plasma | Is a | 33747003 | Glucose measurement, blood |
Table 2. The extract from the hierarchical crosswalk for the generic SNOMED Measurement, which is connected to more granular LOINC Laboratory tests through a “Subsumes” relationship.
| snomed_code | snomed _name | relationship_id | loinc_code | loinc_name |
| 33747003 | Glucose measurement, blood | Subsumes | 93791-2 | Glucose [Mass/volume] mean in Serum or Plasma |
| 33747003 | Glucose measurement, blood | Subsumes | 96799-2 | Glucose [Mass/volume] in Serum or Plasma --30 minutes post dose arginine |
| 33747003 | Glucose measurement, blood | Subsumes | 96800-8 | Glucose [Mass/volume] in Serum or Plasma --1 hour post dose arginine |
| 33747003 | Glucose measurement, blood | Subsumes | 96802-4 | Glucose [Mass/volume] in Serum or Plasma --1.5 hours post dose arginine |
| 33747003 | Glucose measurement, blood | Subsumes | 6762-9 | Glucose [Mass/volume] in Serum or Plasma --1.5 hours post 50 g lactose PO |
| 33747003 | Glucose measurement, blood | Subsumes | 2339-0 | Glucose [Mass/volume] in Blood |
| 33747003 | Glucose measurement, blood | Subsumes | 13865-1 | Glucose [Mass/volume] in Serum or Plasma --2.5 hours post 50 g lactose PO |
| 33747003 | Glucose measurement, blood | Subsumes | 6756-1 | Glucose [Mass/volume] in Serum or Plasma --4.5 hours post 75 g glucose PO |
| 33747003 | Glucose measurement, blood | Subsumes | 12610-2 | Glucose [Mass/volume] in Serum or Plasma --2 hours post XXX challenge |
| 33747003 | Glucose measurement, blood | Subsumes | 27432-4 | Glucose [Mass/volume] in Serum or Plasma --8th specimen post XXX challenge |
| 33747003 | Glucose measurement, blood | Subsumes | 1539-6 | Glucose [Mass/volume] in Serum or Plasma --4 hours post 75 g glucose PO |
| 33747003 | Glucose measurement, blood | Subsumes | 25669-3 | Glucose [Moles/volume] in Serum or Plasma --3.5 hours post dose glucose |
| 33747003 | Glucose measurement, blood | Subsumes | 32359-2 | Glucose [Moles/volume] in Serum or Plasma --10 minutes post dose glucose |
| 33747003 | Glucose measurement, blood | Subsumes | 14743-9 | Glucose [Moles/volume] in Capillary blood by Glucometer |
| 33747003 | Glucose measurement, blood | Subsumes | 1548-7 | Glucose [Mass/volume] in Serum or Plasma --pre 0.5 g/kg glucose IV |
As far SNOMED and LOINC put on hold the collaborative work, the hierarchy can be extended through the creation of hierarchical mappings for those LOINC Parts and SNOMED Attributes which do not exist yet, which can be incorporated into existing SQL-script. However, this approach looks time-consuming. Thus, we plan to review the status of Classification concepts of LOINC Hierarchy, which, becoming standard and being integrated into the LOINC-SNOMED Hierarchy as descendants of generic SNOMED concepts, can bring along all related Standard Measurements.
Quick access:
- Home
- News
- Introduction
- Glossary
- The Vocabulary Team
- Roadmap
- Release Notes
- Upcoming Changes
- Community Contribution
- General Structure, Download and Use
- Domains
- Vocabularies
- Vocabulary Statistics
- Vocabulary Development Process
- Vocabulary Metadata
- Quality Assurance and Control
- Known Issues in Vocabularies
- Articles
- COVID-19 Vocabulary/ETL Instructions
- Historical Versions