| Author: | Stefano |
|---|---|
| category: | Biology, Chemistry |
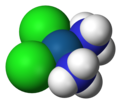
Cisplatin, formula [PtCl2(NH3)2] is a very simple compound of the precious metal platinum. It revolutionized the treatment of some types of cancer, in some cases with almost total chance of success, and it can be considered to full extent the "penicillin for (only some, unfortunately) cancer treatment".
The compound was first synthesized in 1844 by Michel Peyrone, and named after him as Peyrone Chloride, or Peyrone Salt. Yellow in solid form, it produces a transparent, colorless solution when dissolved in water. Nobody suspected its pharmacological activity at that time: it was just one molecule out of many others, a small step in understanding the chemistry of the so-called coordination compounds.
Things changed almost one-hundred year later, and as usually happens for totally fortuitous circumstances. At the beginning of the 60s, Barnett Rosenberg, a physicist-turned-biologist at the Michigan State University, was performing studies on bacterial growth when an electric field is applied, just out of curiosity. He was fascinated by the striking visual similarity between cell replication
and the electric dipole field
Rosenberg curiosity was relative to the effect that an electric field could have on the cell replication. If a dipole was used in the cell to orchestrate the division, an electric field would have interfered. As we are going to see, his experiment will not answer this question (we now know that the cell structure called Centriole has a role on this) but it will lead to a serendipitous and precious discovery.
The plan of action was the following: Rosenberg took a cell growth environment, put two electrodes into it, seeded it with cells, applied electricity and checked the effect on cell duplication. A first test setup with the typical standard bacterium Escherichia coli (the same that lives in our gut) led to longer and longer cells, up to 300 times! Under normal conditions, E. coli grows as a small rod and, after reaching a given length, it divides into two daughter cells; Rosenberg's experiment was blocking the crucial mechanism that allowed the cell to duplicate, so they just kept growing in size.
To find out the culprit of the observed behavior, the first question was: is the applied electric field "per-se" to disrupt the duplication, or some substance that is created inside the cell growth medium? To answer this new question, Rosenberg used the electrodes on a bacterium-free medium, and then transferred the liquid on a bacteria-rich medium. The observed effect: elongation. Clearly, the electric field was not directly responsible. Instead, some long-lived substance generated by the electric field was the actual protagonist, but at the same time this substance was not toxic enough to kill the bacteria. The obvious question became: what was this substance ?
When you apply an electric field to a solution a lot happens at the electrode surface. Electrons are pumped on one electrode and removed from the other, ions migrate and accept or relinquish electrons, new species are created. Taking into account what the solution contained, and following the rules of chemistry Rosenberg and his collaborators Loretta Van Camp and Thomas Krigas checked all the possible outcomes, but none of the potential products were either present or responsible for the observed elongation. We can only imagine how frustrated these poor people could have been, but the truth was very close: having taken everything away the only remaining option, however implausible, must be the right one.
A rather common method to apply an electric dipole to a solution involves platinum electrodes. Platinum is very stable and inert, hence it makes a very nice (but expensive) electrode that does not disturb the solution. This was, at least, the common wisdom for the usual cases. It is not difficult to see how Rosenberg and colleagues never considered this option until the very last, but this was their breakthrough: due to the game between applied potential and experimental setup, the metallic platinum was attacked and slightly dissolved as ions in the solution. Here, these ions reacted with the cell growth environment to produce cisplatin, ready to act on the bacterial cells.
Cisplatin works by entering the cell, then its nucleus (if it has one, E. coli doesn't have one, being a bacterium). Once inside, the chlorine concentration is much lower, and the two chlorine ions in the drug are lost and replaced with a very weak bond with water. Like removing the cap from a glue stick, the platinum is now free to bind strongly with the nitrogen atoms of two consecutive Adenine or Guanine DNA nucleotides, basically sticking them together. The DNA is now irreparably kinked, also due to the action of a High Mobility Group (HMG) protein, a class of proteins involved in transcription, repair and duplication of DNA. HMG strongly binding to the cisplatin/DNA bases block, and the repair mechanism that could resolve the problem is now completely stuck. With such huge roadblock in place, the cell is unable to perform duplication of DNA to produce a daughter cell, grows indefinitely and dies off afterwards.
Cisplatin was therefore able to stop cell duplication in Escherichia coli, but where does the potentiality for anti-cancer properties come in ? We know that normal, sane cells have a stable mechanism preventing them to grow in number without control, and this mechanism keeps us alive and well. If it breaks, troubles begin. Cells unable to contain their division rate are cancerous in nature, and become aggressive for the body, eventually leading to death. Clearly, a possible way to kill these erratic cells is to prevent them to replicate, and Cisplatin was doing exactly this.
Following these findings, experimentation in mice began, and the impressive results encouraged a trial on humans in 1971. The main problem, and one of the reason why their finding was received with skepticism, was that wisdom of the time considered heavy metals too toxic for human drug application, but these effects could be strongly mitigated by providing full hydration to the patient. In other words, have them drink a lot of water. In addition, the very nature of such approach is aspecific: Cisplatin poisons the whole body, but cancer cells are more sensitive to it when compared to sane cells. The latter ones can grow back, but they are depleted during the treatment, producing (among other factors) a large number of mostly temporary but really unpleasant side effects. In this sense, cisplatin is acting like a policeman arresting everybody in the city to be sure to catch the criminal, assuming that those innocent will be able to demonstrate it. The positive results in human trials were followed by the final approval of cisplatin as an effective anticancer drug in 1978.
Barnett Rosenberg died last year, aged 82. A happy man, we know for sure. He saved countless lives.
- http://www.cancer.org/docroot/CRI/content/CRI_2_4_4X_Chemotherapy_29.asp
- http://www.chemocare.com/bio/cisplatin.asp
- http://www.chm.bris.ac.uk/motm/cisplatin/htmlonly/
- http://pubs.acs.org/cen/coverstory/83/8325/8325cisplatin.html
- http://www.powershow.com/view/146de9-NThmY/The_Effects_of_Cisplatin_Binding_to_DNA
- http://www.chem.tamu.edu/rgroup/marcetta/chem489/Presentations/Cisplatin_Final.pdf
- http://www.nature.com/doifinder/10.1038/205698a0